Zeinab Veisi, Norma Alcantar, and Ryan Toomey Department of Chemical and Biomedical Engineering, University of South Florida, Tampa, FL 33620
Hydrogel coatings are a class of tethered or spatially-confined polymers capable of absorbing significant amounts of water. They are further divided into so-called stimuli-responsive hydrogels, which are materials that imbibe or expel water on demand allowing in situ control of properties and the capacity to generate work. The “stimuli-responsive” designation originates from the ability of the hydrogel to sense its physical and chemical environment, process data, and respond, often with strong amplification of the input signal. Thin films of stimuli-responsive polymers have been demonstrated in technologies that benefit from reversible modulation of surface properties, including drug delivery,separati ons, tissue cultures, and chromatography.1-4
Most commonly, such stimuliresponsive hydrogels are derived from petroleum-based monomersthat undergo changes in solubility with either pH or temperature. Poly(N-isopropylacrylamide), or poly(NIPAAm), is a well-known lower critical solution temperature (LCST) polymer that experiences a sharp phase transition when subjected to small perturbations in external stimuli. For example, as shown in Figure 1, hydrogels of poly(NIPAAm) in water undergo a hydrophilic/hydrophobic transition at roughly 32oC.5-6
These types of polyemrstypically comprise moieties that have both hydrophobic and hydrophilic character, such that a small change in the environmental conditions cause a change in the balance between these two characters, either favoring the swollen “hydrophilic” state or the collapsed “hydrophobic” state. At low temperatures, solubility of the macromolecule is engendered through favorable mixing of the hydrophilic moieties and water. As temperature is increased, a point is reached where mixing can no longer stabilize hydrophobic interactions and the polymer phase-separates from solution, leading to a strong collapse in the volume of the hydrogel.
With a growing demand towards replacing petroleum-based polymers with sustainable alternatives, we have explored pectin polysaccharidesto mimic such properties. Pectin polysaccharides are a complex and structurally diverse group of natural polymers that possess hydrophilic and hydrophobic functionality, which could have significant potential as stimuli-sensitive hydrogels. Pectin is a heteropolysaccharide extracted from cell walls and intercellular regions of plants or their fruits (such as cactus mucilage or orange peels7-9) functioning as the hydrating component of the cellulosic network.10-11 In general, the structure is mostly composed of D-galacturonic acid residues linked by α-1,4- glycosidic bonds, which are often interrupted with different sugars such as rhamnose, arabinans, galactose, xylose, etc. The carboxyl group associated with the D-galacturonic acid residue exists in two primary forms: The carboxylate salt and the neutral ester form.12-15 Depending on the pectin source and extraction technique, pectinscan either have a low esterified content (LE, 50% esterification). This opens up non-synthetic routes to tune the response of the pectin coatings based solely on the source and method of extraction.
To illustrate this point, pectin was extracted from two sources: orange peels and Opuntia ficus-indica(OFI) cacti. The OFI cactus (commonly referred to as Nopal or prickly pear) is grown in semi-arid and arid regions as a food source.
The pectin polysaccharides extracted from both orange peels and OFI cacti have a high molecular weight and are capable of holding high volumes of water within the polysaccharide matrices, rendering hydrogels with a multitude applications. However, the pectin from the orange peels is highly esterified with a degree of esterification near 70%, whereas the pectin from OFI cactus has a much lower degree of esterification (near 25 %).
Coatings of each type of pectinpolysaccharide were fabricated by first spin-coating the pectin solution onto a solid surface followed by exposure to a calcium chloride (CaCl2 )/ ethanol solution. Ethanol, a poor solvent for pectin, prevents dissolution of the coating while simultaneously allowing CaCl2 diffusion in order to establish Ca2+ bridges between carboxyl groups to cross-link the pectin coating. The underlying mechanism is understood by the well-known ‘egg-box’ model, which describes the formation of networks through associations between Ca2+ and acidic regions of the pectin.16-19
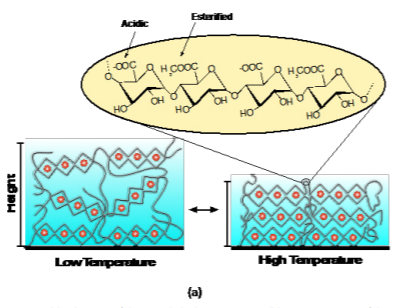
Figure 2(a) shows a schematic of the cross-linked pectin coating and chemical composition. Figure 2(b) shows the attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) spectra of the two types of pectin coatings (extracted from the orange peels and OFI cactus). The two most relevant peaks with respect to the degree of esterification are centered at approximately 1610 cm-1 (corresponding to the carboxylate, or –COO- vibration of the non-esterified galacturonic acid residues) and 1740 cm-1 (corresponding to the carboxylester, –COOR, vibration of the esterified galacturonic acid residues). The bands associated with the esterified residues in OFI cactus pectin are less distinct than the orange peel pectin indicating a lower degree of esterification. The resultant swelling profiles for 100 nm thick films are shown in Figure 2(c). The degree of swelling is defined as the swollen thickness divided by the dry thickness. Interestingly, both types of pectin have a prominent deswelling transition as temperature is increased above 30 ˚C, which we have shown to be driven by the dehydration of the Figure 2. (a) Schematic of the cross-linked pectin coating; (b) ATR-FTIR spectra of the pectin coatings based on orange peels and OFI Cactus; (c) Swelling profiles of the pectin coatings as a function of temperature.
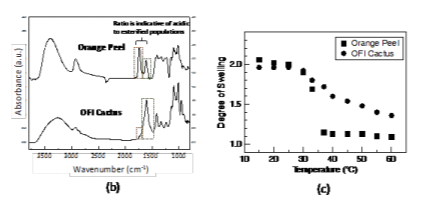
esterified galacturonic acid residues.20 The transition is stronger in the orange peel pectin due to the higher degree of esterification, providing a mechanism to tune the transition depending solely on degree of esterification.
As a demonstration of the thermal response of the pectin coatings, Figure 3 shows the ability of the orange peel pectin coating to culture and release NIH3T3 mouse embryonic fibroblast cells. When cultured at 37 ˚C, the coating is collapsed providing an adhesive surface for cells to attach and spread. Upon lowering the culture temperature to 20 ˚C, the coating swells and releases the attached cells. After 60 min of incubation at 20 ˚C, the majority of the cells are detached from the coating.
In conclusion, pectin extracted from natural sources shows promise as responsive hydrogel coatings, which has the potential to replace petroleum-derived materials. The degree of esterification furthermore allows control over the response, which opens up avenues to control properties of the coating solely through the source and type of extraction process without having to resort to additional synthesis.
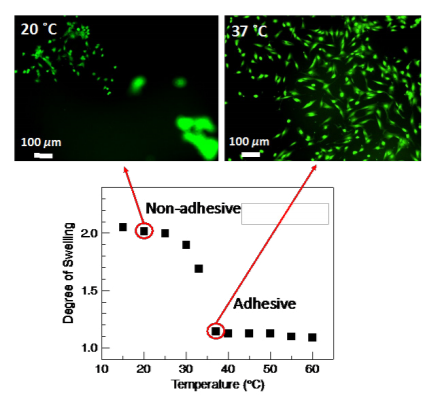
Figure 3. A pectin coating is adhesive to cells in the collapsed state (37 ˚C) and repulsive to cells in the swollen state (20 ˚C), functioning as an on-off tissue culture platform.
references
(1) Hoffman, A. S., Environmentally Sensitive Polymers and Hydrogels - Smart Biomaterials. Mrs Bull 1991,16, 42-46.
(2) Huber, D. L.; Manginell, R. P.; Samara, M. A.; Kim, B. I.; Bunker, B. C., Programmed Adsorption and Release of Proteins in a Microfluidic Device. Science 2003,301, 352-354.
(3) Kikuchi, A.; Okano, T., Intelligent Thermoresponsive Polymeric Stationary Phases for Aqueous Chromatography of Biological Compounds. Progress In Polymer Science 2002,27, 1165.
(4) Kushida, A.; Yamato, M.; Konno, C.; Kikuchi, A.; Sakurai, Y.; Okano, T., Decrease in Culture Temperature Releases Monolayer Endothelial Cell Sheets Together with Deposited Fibronectin Matrix from TemperatureResponsive Culture Surfaces. Journal of Biomedical Materials Research 1999,45, 355-362.
(5) Schild, H. G., Poly (N-Isopropylacrylamide) - Experiment, Theory and Application. Progress in Polymer Science 1992,17, 163-249.
(6) Shibayama, M.; Tanaka, T., Volume Phase-Transition and Related Phenomena of Polymer Gels. Adv Polym Sci 1993,109, 1-62.
(7) Voragen, A. G.; Coenen, G.-J.; Verhoef, R. P.; Schols, H. A., Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Structural Chemistry 2009,20, 263.
(8) Goycoolea, F. M.; Cárdenas, A., Pectins from Opuntia Spp.: A Short Review. Journal of the Professional Association for Cactus Development 2003,5, 17-29.
(9) Maran, J. P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R., Optimization of Microwave Assisted Extraction of Pectin from Orange Peel. Carbohydrate polymers 2013,97, 703- 709.
(10) Thakur, B. R.; Singh, R. K.; Handa, A. K.; Rao, M., Chemistry and Uses of Pectin—a Review. Critical Reviews in Food Science & Nutrition 1997,37, 47-73.
(11) Burton, R. A.; Gidley, M. J.; Fincher, G. B., Heterogeneity in the Chemistry, Structure and Function of Plant Cell Walls. Nature Chemical Biology 2010,6, 724-732.
(12) Ridley, B. L.; O'Neill, M. A.; Mohnen, D., Pectins: Structure, Biosynthesis, and Oligogalacturonide-Related Signaling. Phytochemistry 2001,57, 929-967.
(13) Willats, W. G.; Knox, J. P.; Mikkelsen, J. D., Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends in Food Science & Technology 2006,17, 97-104.
(14) Yapo, B. M., Pectic Substances: From Simple Pectic Polysaccharides to Complex Pectins—a New Hypothetical Model. Carbohydrate Polymers 2011,86, 373-385.
(15) Mohnen, D., Pectin Structure and Biosynthesis. Current opinion in plant biology 2008,11, 266-277.
(16) Powell, D.; Morris, E.; Gidley, M.; Rees, D., Conformations and Interactions of Pectins: Ii. Influence of Residue Sequence on Chain Association in Calcium Pectate Gels. Journal of molecular biology 1982,155, 517-531.
(17) Walkinshaw, M.; Arnott, S., Conformations and Interactions of Pectins: Ii. Models for Junction Zones in Pectinic Acid and Calcium Pectate Gels. Journal of Molecular Biology 1981,153, 1075-1085.
(18) Axelos, M.; Thibault, J., The Chemistry of Low-Methoxyl Pectin Gelation. The chemistry and technology of pectin 1991,6, 109- 108.
(19) Grant, G. T.; Morris, E. R.; Rees, D. A.; Smith, P. J.; Thom, D., Biological Interactions between Polysaccharides and Divalent Cations: The Egg-Box Model. FEBS letters 1973,32, 195-198.
(20) Veisi, Z.; Gallant, N. D.; Alcantar, N. A.; Toomey, R. G., Responsive Coatings from Naturally Occurring Pectin Polysaccharides. Colloid Surface B 2019,176, 387-393.




